Lifeasible focuses on key factors in plant-pathogen interactions and offers services to help explore the role of protein glycosylation in plant-pathogen interactions.
Protein glycosylation is a kind of common post-translational modification of proteins that can alter protein conformation, activity, and stability. Substrates for protein glycosylation are mainly hexoses and their derivatives, such as D-glucose, D-galactose, D-mannose, and fucose. Sialic acid or xylose are also substrates for the glycosylation of some proteins. When protein glycosylation occurs in plant-pathogen interactions, it can affect host resistance or pathogen virulence. For example, glycosylation by plants stabilizes plant extracellular proteinases and regulates their pathogen resistance. Fungi prevent plant recognition by glycosylating their LysM effectors for ultra-high chitin affinity. Lifeasible helps research the role of protein glycosylation in plant pathogens and provides professional research services.
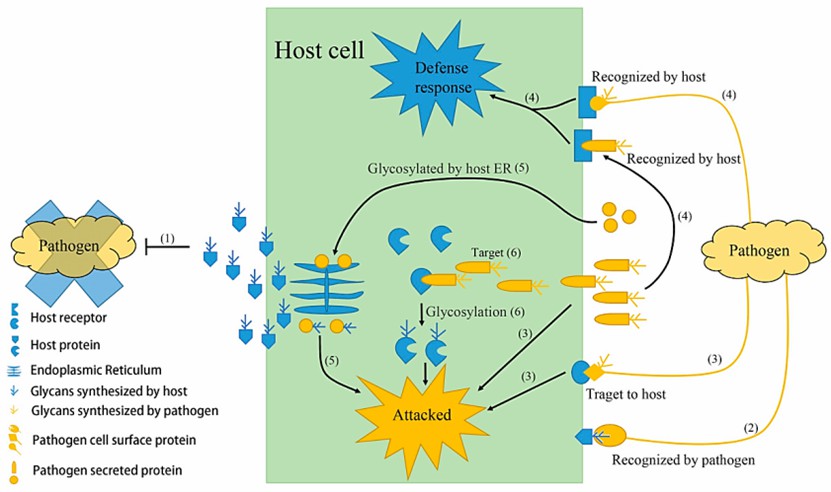 Fig. 1 Schematic depicting the multiple functions of glycoproteins in host-pathogen interaction (Lin et al., 2020).
Fig. 1 Schematic depicting the multiple functions of glycoproteins in host-pathogen interaction (Lin et al., 2020).
Detection of protein glycosylation
We help analyze the glycosylation of plant or pathogen proteins to help determine if it is protein glycosylation that affects plant-pathogen interactions.
We help enrich proteins and prepare samples for glycosylation analysis. Our commonly used methods for enriching glycoproteins include the lectin-based affinity technique, hydrazine chemistry-based technique, hydrophilic interaction chromatography, and the β-elimination method. For glycoproteins to be tested where enrichment methods are lacking, we can help try multiple enrichment approaches.
We help to identify protein glycosylation. For identifying N-glycosylated proteins, our commonly used methods include the PNGase F enzyme method and the Endo H enzyme method. Among them, the Endo H enzyme method is used for heterotrimeric glycoproteins and high-mannose-type glycoproteins. For O-glycosylated proteins, we commonly use the chemical process. We also use trifluoromethanesulfonic acid (TFMS) to achieve glycosylation site analysis.
We help analyze the structure of glycans by mass spectrometry (MS) and nuclear magnetic resonance (NMR) spectrometry. We use matrix-assisted laser desorption/ionization (MALDI)-time-of-flight (TOF) MS to analyze the structure, which is highly sensitive for the structural characterization of glycosylated compounds and allows for high throughput detection. We use NMR for stereochemical structure analysis of glycan chains.
Exploring the role of protein glycosylation
We help to perform glycoproteome analysis to determine the glycosylation produced in plant-pathogen interactions and help in the initial exploration of the role of protein glycosylation.
We help to use RNA silencing to explore the role of glycosylated proteins. We help with the heterologous expression of glycosylated or unglycosylated proteins to study the subcellular localization of proteins to determine the direction of action studies. We further help explore the effect of glycosylated proteins on plant-pathogen interactions, including effects on plant-pathogen recognition, plant immunity, pathogen virulence, evasion immunity of pathogen, and plant resistance to pathogen invasion. In addition, we also help to detect the effect of different glycosylation on plant-pathogen interactions. If there is a need to explore the location where glycoproteins achieve glycosylation, we can achieve that as well.
The research directions of protein glycosylation in plant-pathogen interactions are extensive. Lifeasible will tailor services based on the results of preliminary experiments and the needs of our customers. If you have a research need for protein glycosylation in plant-pathogen interaction, please contact us.
References
Lifeasible has established a one-stop service platform for plants. In addition to obtaining customized solutions for plant genetic engineering, customers can also conduct follow-up analysis and research on plants through our analysis platform. The analytical services we provide include but are not limited to the following:
July 13, 2024