Non-repetitive terminal structural domains have been identified at the N and C ends of spider silk proteins, which are thought to have essential functions for protein assembly. Spider silk contains large amounts of glycine and alanine, which require an additional metabolic pool of amino acids when expressed by fast-growing microorganisms, and the highly repetitive gene sequences of spider silk protein are prone to recombination and gene instability. Since plants can synthesize amino acids from the initial raw material and the spider silk protein genes transferred into plants are less susceptible to recombination loss, with the development of plant bioengineering technology, plants can be used as vectors to produce spider silk proteins. By transplanting synthetic genes for spider silk protein production into plants, such as peanuts, tobacco, and cereals, these plants can produce spider silk-like proteins in large quantities, which can then be extracted for spinning.
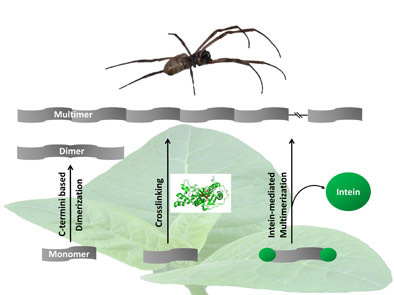 Fig.1 Spider silks from plants. (Hauptmann et al., 2013)
Fig.1 Spider silks from plants. (Hauptmann et al., 2013)
Lifeasible offers professional services for the synthesis of spider silk proteins from plants. Our researchers can customize a proprietary solution for your project and help you complete your product development as soon as possible.
1. Non-repetitive C-terminal dimerization
Spider silk proteins are produced by flagellated glands containing non-repetitive C-termini. These C-termini are used to produce spider silk protein dimers in plants.
2. Transglutaminase cross-linking
Specific crosslinking of suitable amino acid motifs can allow defined protein dimerization and/or multimerization. Here, transglutaminase performs this task as a catalase, forming a covalent bond between the free amine group (peptide or protein-bound lysine) and the γ-carboxamide group of the peptide or protein-bound glutamine. In transgenic plants, lysine and internal glutamine in the C-terminal region can cause multiple cross bonds, leading to nonlinear linkage and crossover of peptide chains and the formation of nonlinear multimers.
3. Intrinsic peptide-mediated multimerization
Multimerization based on intracellular processes is advantageous because auxiliary processing and additional supplementation are unnecessary. Mutagenesis of critical amino acids to prevent multimerization of siderophore protein monomers in different independent transgenic plants. This approach represents a new pathway that can be used not only to produce high molecular weight spider silk proteins larger than 250 kDa but also to produce a wide range of different repeat proteins. Intercrossing different transgenic strain pairs provides an additional plant-specific approach to producing composites. The intrinsic peptide-mediated multimeric structures form microfibrils with potential use as biomaterials.
If you are interested in our services, or if you want a service not listed above, please feel free to contact us, and our staff will customize a professional product solution for you.
Reference
Lifeasible has established a one-stop service platform for plants. In addition to obtaining customized solutions for plant genetic engineering, customers can also conduct follow-up analysis and research on plants through our analysis platform. The analytical services we provide include but are not limited to the following:
July 13, 2024