Microbial expression systems have the advantages of fast host growth, high yield, short cycle time, simple culture conditions, clear genetic background, and various expression vectors, strains, and purification systems. The microbial expression strategy is to obtain spider silk proteins through bacterial fermentation by recombining or doubling different repetitive sequences and cloning them into the expression vector. Based on our professional staff and laboratory equipment, Lifeasible can provide our customers with professional microbial expression strategies to prepare spider proteins.
When using E. coli to express spider silk-like proteins, due to different codon preferences from spiders and the removal of repetitive sequences by homologous recombination in the host bacteria, the spider silk protein genes are prone to premature termination of translation and internal deletion or mismatching of genes encoding spider silk protein repetitive sequences, resulting in genetic instability and a decrease in recombinant protein yield as the molecular mass of spider silk protein increases. In the yeast expression system, although there is no gene deletion, the expression of secretory proteins is not efficient. The expression of non-secretory proteins is significantly affected by gene copy number, and the yield of large molecular mass spider proteins is unsatisfactory. To obtain recombinant spider proteins with high yield and large molecular mass through the microbial pathway, we need to continue the expression of exogenous genes by optimizing genetic information and adjusting bacterial codon preference or continue to search for more efficient expression vectors to express spider proteins.
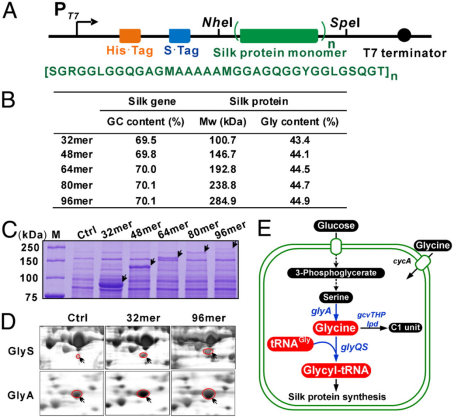 Fig.1 Recombinant expression of spider traction silk protein in Escherichia coli. (Xia et al., 2010)
Fig.1 Recombinant expression of spider traction silk protein in Escherichia coli. (Xia et al., 2010)
Lifeasible can provide you with solutions for preparing arachidins from E. coli and other types of microorganisms, such as yeast. The following are the pathways for the preparation of arachnoproteins in E. coli.
E. coli cells are grown at 30 °C. Expression experiments were performed in medium, except for HCDC (high cell density culture). Cells were induced and used for 2D gel electrophoresis, SDS-PAGE, and plasmid copy number analysis. Cells were collected and stored.
HCDC of recombinant E. coli was performed in a fermenter-containing medium supplemented with glucose. The dissolved oxygen concentration was maintained at 40% of air saturation. Cells were induced when OD600 reached ~60.
The cell suspension was stirred, then pH adjusted with glacial acetic acid and incubated for an additional 2 h at room temperature under magnetic stirring. After centrifugation, the solvent-precipitated supernatant was added and incubated with stirring. The mixture was centrifuged, and the resulting supernatant was precipitated. The precipitate was dissolved and dialyzed. After centrifugation, freeze dry.
Freeze-dried silk proteins were dissolved in a solvent to prepare the spinning stock solution. After spinning, the primary spun fibers are held in a coagulation bath. The fibers are dried to prevent shrinkage and to maintain the elongation length for measurement. Tensile tests are performed using a universal tensile tester with a load cell.
Observe the surface and cross-section of the fiber through a field emission scanning electron microscope. The fracture surface is observed after the tensile test in the cross section, while the original filament is used for surface observation.
If you are interested in our services, or if you want a service not listed above, please feel free to contact us, and our staff will customize a professional product solution for you.
Reference
Lifeasible has established a one-stop service platform for plants. In addition to obtaining customized solutions for plant genetic engineering, customers can also conduct follow-up analysis and research on plants through our analysis platform. The analytical services we provide include but are not limited to the following:
July 13, 2024