Recent breakthroughs in transgenic technology, especially those based on the CRISPR/Cas system, have greatly boosted research in plant biology, agriculture, and biotechnology. CRISPR-mediated gene editing, with its high precision and versatility, has great potential for accelerating basic plant science research and crop improvement through the creation of multiple types of genetic variants and functionally optimized gene alleles. It has great potential for accelerating basic plant science research and crop improvement. The first step in plant transformation is the delivery of exogenous DNA into recipient cells. This step in plant gene editing enables the expression and function of CRISPR/Cas reagents inside the cell, including single-guide RNA (sgRNA) and Cas DNA nuclease. This involves a lengthy, expensive, and labor-intensive tissue culture step and is currently only possible in a limited number of plant species, making it a significant bottleneck in plant gene editing.
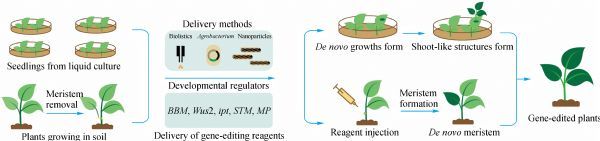 Fig. 1. Gene-edited plants produced by de novo meristem induction. (ZHANG Ji et al., 2020)
Fig. 1. Gene-edited plants produced by de novo meristem induction. (ZHANG Ji et al., 2020)
To alleviate the bottleneck posed by tissue culture-based genetic transformation, our breeders are committed to developing tissue culture-free gene editing solutions for plants. Lifeasible offers promising methods for producing gene-edited plants by utilizing the de novo meristem induction and simultaneously avoiding tissue culture. This approach can significantly increase the utility of gene editing in plants.
By reprogramming genome-edited somatic cells into meristematic tissues through co-expression of developmental regulatory factors (dr) and gene editing components, thereby simplifying or completely avoiding tissue culture and further enabling direct regeneration of somatic gene-edited plants. The specific processes are as follows:
(1) We have successfully established the Fast-Treated Agrobacterium co-culture (Fast-TrACC) method to deliver DNA constructs into plant cells by transient expression and use luciferase as a reporter gene.
(2) Multiple promoters were used in different combinations to ectopically express dr appropriately in plants (Bensheim's tobacco, potato, grapevine), including WUSCHEL2 (Wus2) and SHOOT MERISTEMLESS (STM) or MONOPTEROS (MP) to induce the formation of meristem-like structures.
(3) Using the same approach, a single guide RNA (sgRNA) targeting the two tested genes was introduced into the leaves of transgenic plants, constitutively expressing Cas9 with a successful combination of developmental regulators.
(4) Recovery of gene-edited plants by culturing shoots on rooting medium.
The above scheme can produce gene-edited shoots but requires a rooting step. We are also working to develop a new protocol to directly induce gene-edited shoots using soil-grown cas9-expressing plants that will grow and produce seeds with the target mutation. Agrobacterium cultures carrying dr and expressing sgRNA constructs were injected into pruned sites where naturally developing meristematic tissue had been removed to accomplish this.
Lifeasible is committed to providing rapid and effective tissue culture-free gene editing solutions to customers worldwide. Our solution makes it possible to perform high-throughput plant genetic modification economically, thus accelerating the dissection of gene functions and the improvement of agronomic traits using artificially edited superior alleles. Contact us today to learn more about our solutions.
Reference
Lifeasible has established a one-stop service platform for plants. In addition to obtaining customized solutions for plant genetic engineering, customers can also conduct follow-up analysis and research on plants through our analysis platform. The analytical services we provide include but are not limited to the following:
July 13, 2024