Drosophila belongs to the group of small flies with a body length between 1.5 and 4 mm. The body is mostly yellowish-brown, but some are black. The head has a pair of large, mostly bright red compound eyes.
Drosophila has a high degree of genetic similarity to mammals, while it is relatively simple yet can perform complex behaviors, and has been used extensively in genetic and developmental biology studies, making it an ideal model organism for research.
Drosophila melanogaster, the representative species of Drosophila, is widely used in genetic studies. Drosophila melanogaster is a dipteran insect with a short life history, easy rearing, fast reproduction, few chromosomes, many mutant types, and small individuals, making it an excellent material for genetic experiments and a commonly used model organism.
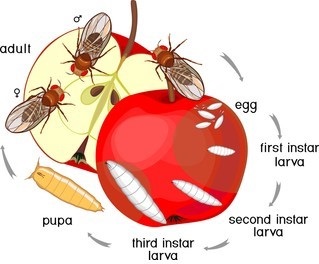 Figure 1. Typical Drosophila life cycle.
Figure 1. Typical Drosophila life cycle.
TALEN is more specific for DNA recognition and less cytotoxic compared to ZFN. Lifeasible has established a method for Drosophila gene knockdown using TALEN. We can successfully knock out the target gene by injecting mature mRNA encoding TALEN directly into Drosophila (F0) embryos, and can also perform double knockout.
Of course, the application of TALEN in Drosophila is not limited to mutant production, but can also be applied to many forms of gene editing. For example, we can combine TALEN protein with transcriptional regulators to regulate the expression of target genes.
Compared to Ends-in approach-mediated gene mutation, ZFN-mediated gene-editing technology is an efficient and relatively simple process in Drosophila mutant fabrication. Lifeasible can successfully obtain Drosophila mutants by directly injecting mature mRNA encoding ZFN into Drosophila embryos. This approach not only simplifies the experimental procedure and improves the efficiency of conventional ZFN targeting, but also makes it possible to use ZFN for knocking out large segments of the Drosophila genome or for simultaneous multiple knockouts.
Gene knockout is a classical reverse genetics approach. To obtain efficient and heritable Drosophila mutants, Lifeasible uses CRISPR/Cas9 technology combined with Homology Directed Repair (HDR) strategy to edit the Drosophila genome, which can knock out target genes or introduce molecular modifications according to design. Our CRISPR/Cas9 system-based gene-editing technology offers the advantages of being simple and specific, efficient and cost-effective.
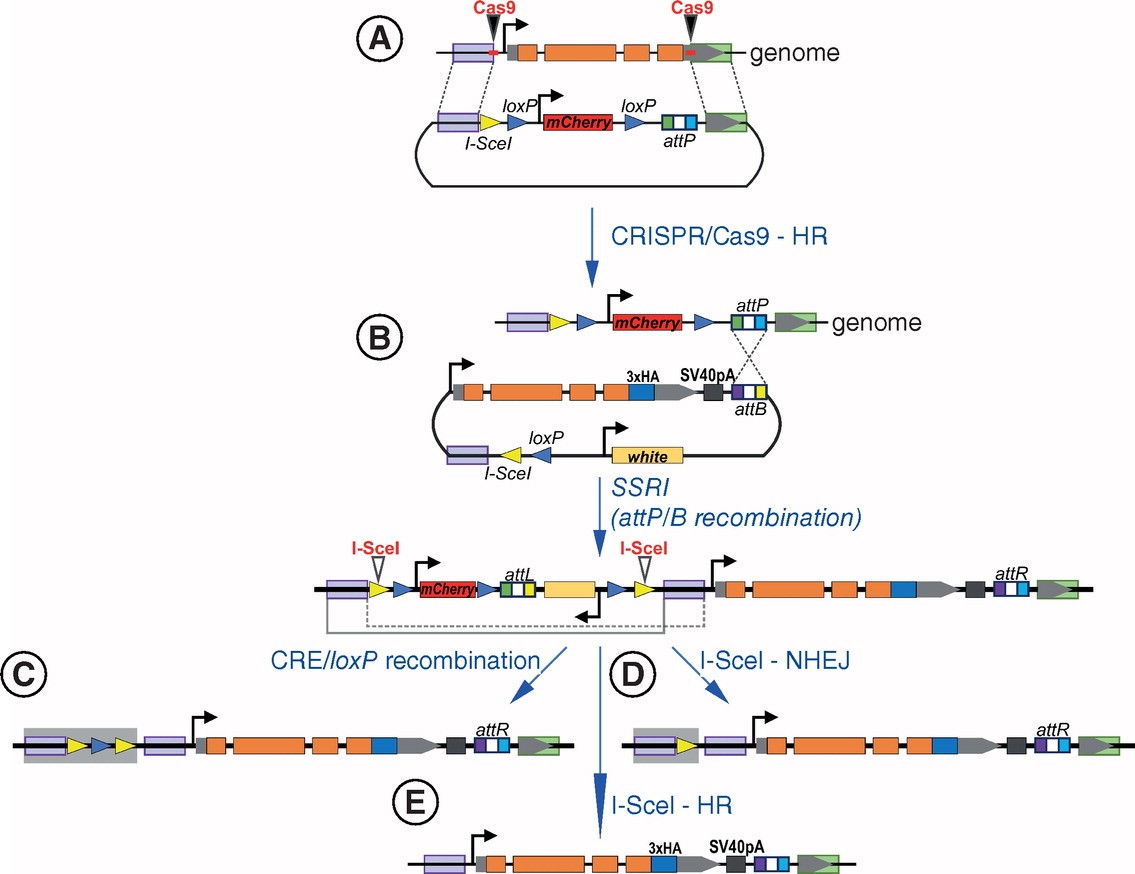 Figure 2. Scheme of gene editing using I-SceI endonuclease in combination with CRISPR/Cas9 and site-specific recombinase-mediated integration techniques. (Nikolay Z., et al., 2019)
Figure 2. Scheme of gene editing using I-SceI endonuclease in combination with CRISPR/Cas9 and site-specific recombinase-mediated integration techniques. (Nikolay Z., et al., 2019)
We use CRISPR/Cas9 not only for targeted gene knockout but also for other applications such as conditional knockout of genes, large fragment knockout of genes, CRISPRi, etc. conveniently.
Using gene-editing technologies such as CRISPR/Cas9, TALEN, and ZFN, Lifeasible enables a variety of precise edits to the Drosophila genome, including:
References:
Lifeasible has established a one-stop service platform for plants. In addition to obtaining customized solutions for plant genetic engineering, customers can also conduct follow-up analysis and research on plants through our analysis platform. The analytical services we provide include but are not limited to the following:
July 13, 2024